Acidic Hydrolysis of an Amide Produces Which of the Following
The hydrolysis of an amide produces an organic acid and ammonia. Or if we wanted to form a carboxylic acid first thing we would do is sodium hydroxide and heat things up Na plus OH minus once again heat things up.
Acidic Hydrolysis Of Amides Master Organic Chemistry
H2O H2O Write the amide hydrolysis reaction for the following amides.

. Ester hydrolysis occurs relatively easily but. Amide hydrolysis produces the same. Correct option is B As shown in the above reaction acetic acid is formed on complete hydrolysis of acetonitrile.
Acidic and Basic Ami Hydrolysis E. 3 All cyclic amines are heterocyclic compounds that are either 10 or 20 amines. Amide hydrolysis produces the same product under all conditions.
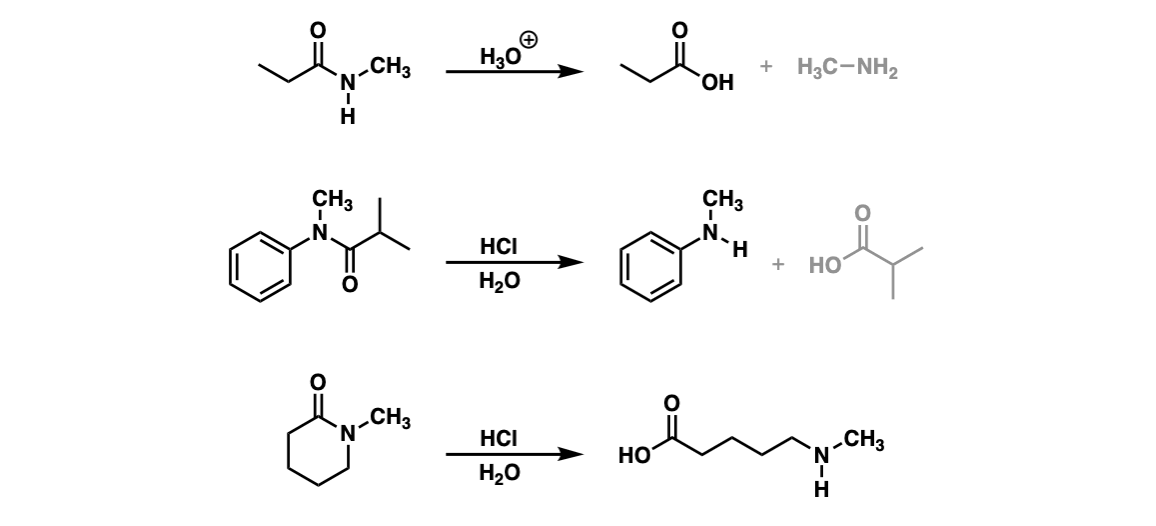
The carbonyl oxygen is lost in amide hydrolysis. The hydrolysis of an amide produces an organic acid and ammonia. RC ON R2 OH RC OO H N R2.
Amide hydrolysis occurs spontaneously when amide is dissolved in water The mechanism of amide hydrolysis is the same as that of ester hydrolysis. Acetaminophen is an amide. A All three statements are true.
The reaction resembles ester hydrolysis but there are important differences. Which one of the following functional groups undergoes hydrolysis with alkali to yield an acid group. That of course would give us a carboxylate anion so we need to protonate in the second step to form benzoic acid as our product.
View solution Which of the following is hydrolysed to give secondary amine. Hydrolysis of Amides Amides are very stable in water. Acidic hydrolysis gives a carboxylic acid and an amine salt.
Peptides and the Peptide Bond. The unstable intermediate is stabilized by the loss of ammonia. Butyramide thus yields butyric acid and ammonia.
View solution Hydrolysis of alkyl isocyanide yields. Acidic hydrolysis involves the nucleophilic addition of water to the protonated amide. 1 Acidic hydrolysis of an amide produces an acid salt and an amine.
Draw the molecular structure of the amine that is formed in the following reaction. 2004 2133 Place a line through each bond of distamycin that would be cleaved by acid hydrolysis. Ester amide acid anhydride thioester What kind of reaction occurs between an acid halide and water to produce a carboxylic acid and a halogenic acid.
Amide ester carboxylic acid alcohol What compound is produced in the aminolysis of an acetyl chloride. In aqueous base the products of hydrolysis are a carboxylic acid salt and ammonia or an amine. C Only one of the statements is true.
Which of the following compounds will produce an ester after addition to an acid anhydride. 2 The amide urea contains more nitrogen atoms than carbon atoms. Of course the potential carboxylic acid is a much stronger acid than the neutral amine.
Tylenol an aspirin substitute contains acetaminophen. Butyramide thus yields butyric acid and ammonia. 63 Provide the major organic product in the reaction of CH 3 CH 2 CONH 2 with hot aqueous acid.
16 76 Hydrolysis of Amides The reversibility of this reaction means that an amide can hydrolyze to form an amine and a carboxylic acid. Example of Acid Hydrolysis of Amide. Base Hydrolysis of Amides Draw the condensed structural formulas and give the IUPAC names for the products from the hydrolysis of N- methylpentanamide with NaOH.
However it has little anti-inflammatory effect. Amides undergobase hydrolysis with heat to produce a carboxylate salt and an amine or ammonia. CH_3COcolorredNH_2H_2OHcolorbrownCl rarr CH_3COcolorblueOHcolorredNHcolorbrown4Cl.
1 Acidic hydrolysis of an amide produces an acid salt and an amine. Or we could use base. Benzamide thus yields benzoic acid and ammonia.
62 Distamycin and derivatives have exhibited antiviral antibiotic and antitumor activity by binding to the minor groove of DNA J. Learning Goal Write the common and IUPAC names for amides and the products of formation and hydrolysis. Acetonitriles on hydrolysis produce carboxylic acids with the evolution of ammonia Acetonitrile C H 3 C N H X 2 O.
12 Which of the following bonds has partial double bond character. Thats acid-catalyzed amide hydrolysis. Hence the correct option is B.
A an alcohol and an amine B an amine and a carboxylic acid C a carboxylic acid and an anhydride D an amide and a carboxylic acid E an alcohol and an amide Difficulty. Which of the following statements about amide hydrolysis is correct. In basic hydrolysis the hydroxide ion reacts with the amide.
2 The amide urea contains more nitrogen atoms than carbon atoms. 11 Hydrolysis of a peptide bond produces. In general the hydrolysis of a primary amide forms a carboxylic acid and ammonia or amine.
It acts to reduce fever and pain. Position of Protonation-Several authors80aazs have suggested that the acid- catalysed hydrolysis of amides may. The hydrolysis of a simple amide produces an organic acid and ammonia.
3 All cyclic amines are heterocyclic compounds that are either 10 or 20 amines. Hydrolysis requires the heating of the amide in the presence of strong acid or base. B Two of the three statements are true.
The hydrolysis of a simple amide produces an organic acid and ammonia. This reaction is reversible exergonic. Hydrolysis of an amide breaks the carbonnitrogen bond and produces a carboxylic acid and either ammonia or an amine.
Benzamide thus yields benzoic acid and ammonia. This is followed by the loss of an amine.

18 6 Hydrolysis Of Amides In A Reverse Reaction Of Amidation Hydrolysis Occurs When Water And An Acid Or A Base Split An Amide Learning Goal Write Ppt Download
No comments for "Acidic Hydrolysis of an Amide Produces Which of the Following"
Post a Comment